LDPE is defined by a density range of 09100940 gcm3. This test method covers the determination of the density relative density and API gravity of crude oils that may be handled generally as liquids at test temperatures between 15 C and 35 C utilizing either manual or.
What would be the maximum theoretical percent recovery from the crystallization of 50 g of acetanilide from 100 ml water.
. The molecular weight was calibrated by the standard polystyrene references. Molecular weights of ether and fluorine-based PAA were measured by a. In energy storage applications the energy density relates the energy in an energy store to the volume of the storage facility eg.
The higher the energy density of the fuel the more energy may be stored or transported for the same amount of volume. The solubility of acetanilide in hot water 55 g100 ml at 100 o C is not very great and its solubility in cold water 053 g 100 ml at 0 o C is significant. To remove dicylcohexy ether completely a second distillation of the product is usually carried out.
It is also used as a detergent a fuel insecticide in paints and. 161 into a 50 mL round bottom flask small neck and cautiously add 85 phosphoric acid 3 mL. D 0774 gmL 1.
The value was calculated from the following equation. Breakthrough tests in dry air showed a capacity of 20 to 25 mg of each. The intrinsic viscosity of the PAAs η was confirmed by an Ostwald viscometer at 30 C.
The molecule consists of six-carbon cyclic molecule with a ketone functional groupThis colorless oily liquid has an odor reminiscent of acetoneOver time samples of cyclohexanone assume a pale yellow color. D 0743 gmL and Stoddard Solvent Fisher Scientific Co. Standard Test Method for Density Relative Density and API Gravity Of Crude Oils By Digital Density Analyser.
D 0893 gmL VM. LDPEs intermolecular forces are weaker its tensile strength is lower and its. BP 159 to 176 C.
η ln η. It is not reactive at room temperatures except by strong oxidizing agents and some solvents cause swelling. Cool the cylinder by standing it in a.
Density gcm3 at Temperature C Acetic Acid. 2CH 3 CH 2 OH 2H 2 SO 4 CH 3 CH 2 2 O H 2 SO 4 H 2 Othe temperature of the reaction is carriedout at about 140C to control for unwanted productsthe volatile ether is distilled from themixture. Lets convert the density figure to grams per cubic centimeter gcm 3.
100 g 106 mL bp. Given the high energy density of gasoline the exploration of alternative media to store the energy of powering a car. It is flexible and tough but breakable.
If we want to convert 10mg of sunflower oil to ml then we would do the following. It can withstand temperatures of 80C continuously and 95 C for a short time. Properties of water include its chemical formula H2O density melting boiling point how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom.
Should the density not be in mgml we need to do a little more work. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Converting mg to ml - example 2.
Eluted with 001 M LiBr and H 3 PO 4 DMF solution at a rate of 10 mLmin. Density Test Standard. Petroleum ether can extract stigmasterol and beta-sitosterol from the aerial parts of Ageratum conyzoides and compounds with immunomodulatory activity from Anacyclus pyrethrum.
Ether is produced by the dehydration of ethanol using sulfuric acid. Youll have noticed that the density. To prepare the cell lysate cell pellet of E.
Add 3 boiling chips and arrange for a distillation using a cooled 10 mL graduated cylinder as a receiver. Overall precision and recovery were as shown below representing a non-significant bias in each method. Coli BL21DE3 was resuspended in 20 mL of the lysis buffer lysed by sonication on ice and centrifugated at 14000 rpm for 10 mL.
Cyclohexanone is the organic compound with the formula CH 2 5 CO. Assuming the solution is chilled at 0 o C. Density 077 gmL at 20 C.
17 mL 1 mgmL. Liter to Kilograms Converter l to kg Conversion Liquid density charts. Sunflower oil commonly has a density of 920kgm 3.
Ether can also be prepared by Williamson synthesis. Petroleum ether is used in pharmaceutical industries as a solvent to isolate compounds eg. Learn about its physical chemical properties of water its importance for the existence of life.
BP 120 to 147 C.
Diethyl Ether C4h10o Chemspider

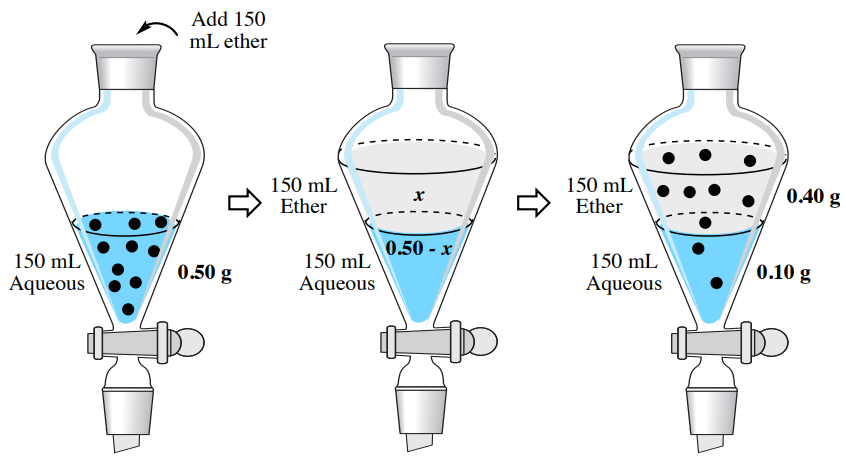
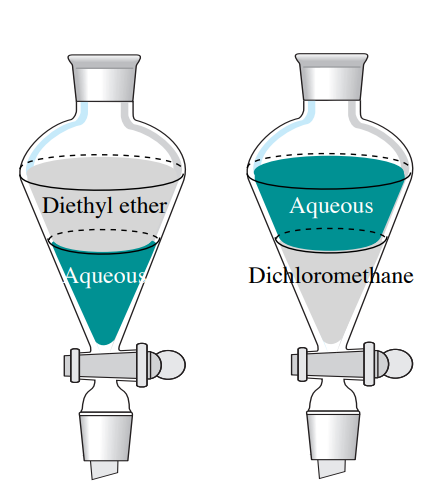
4 5 Extraction Theory Chemistry Libretexts


0 Comments